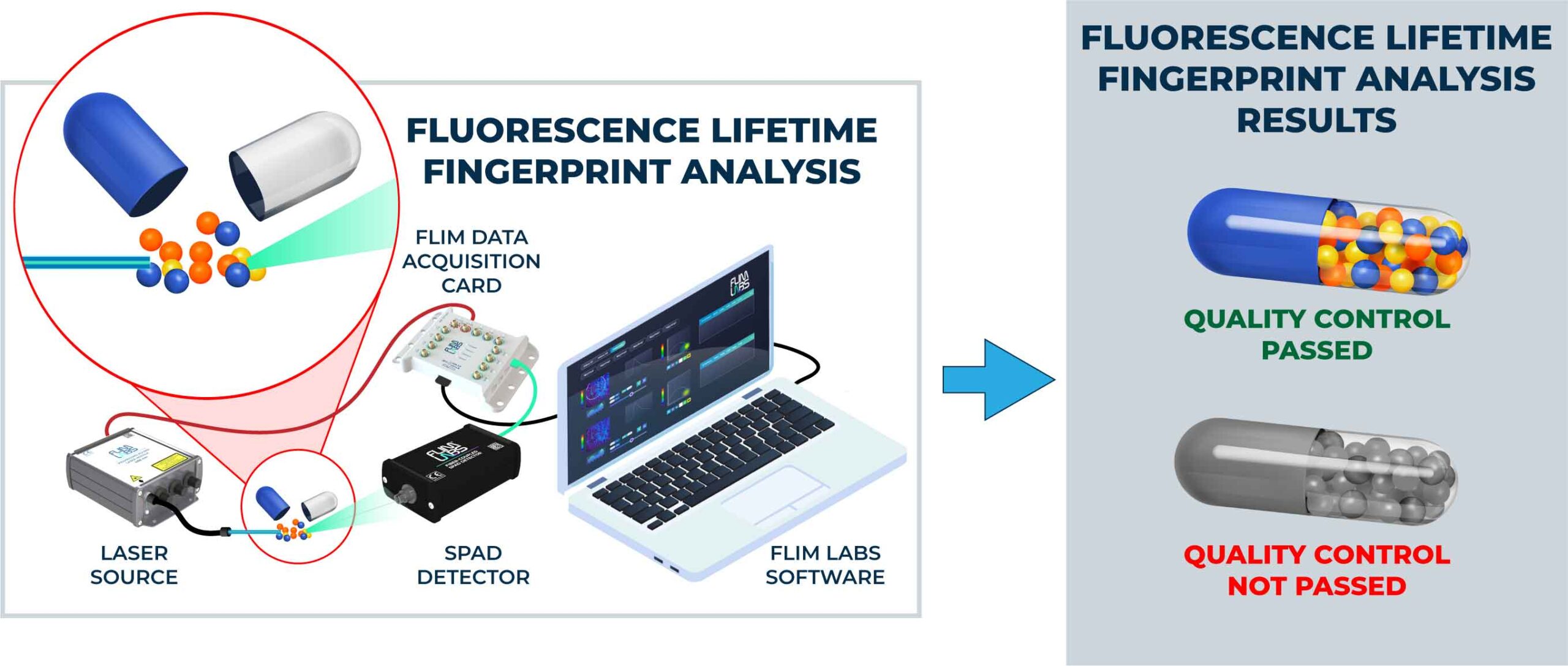
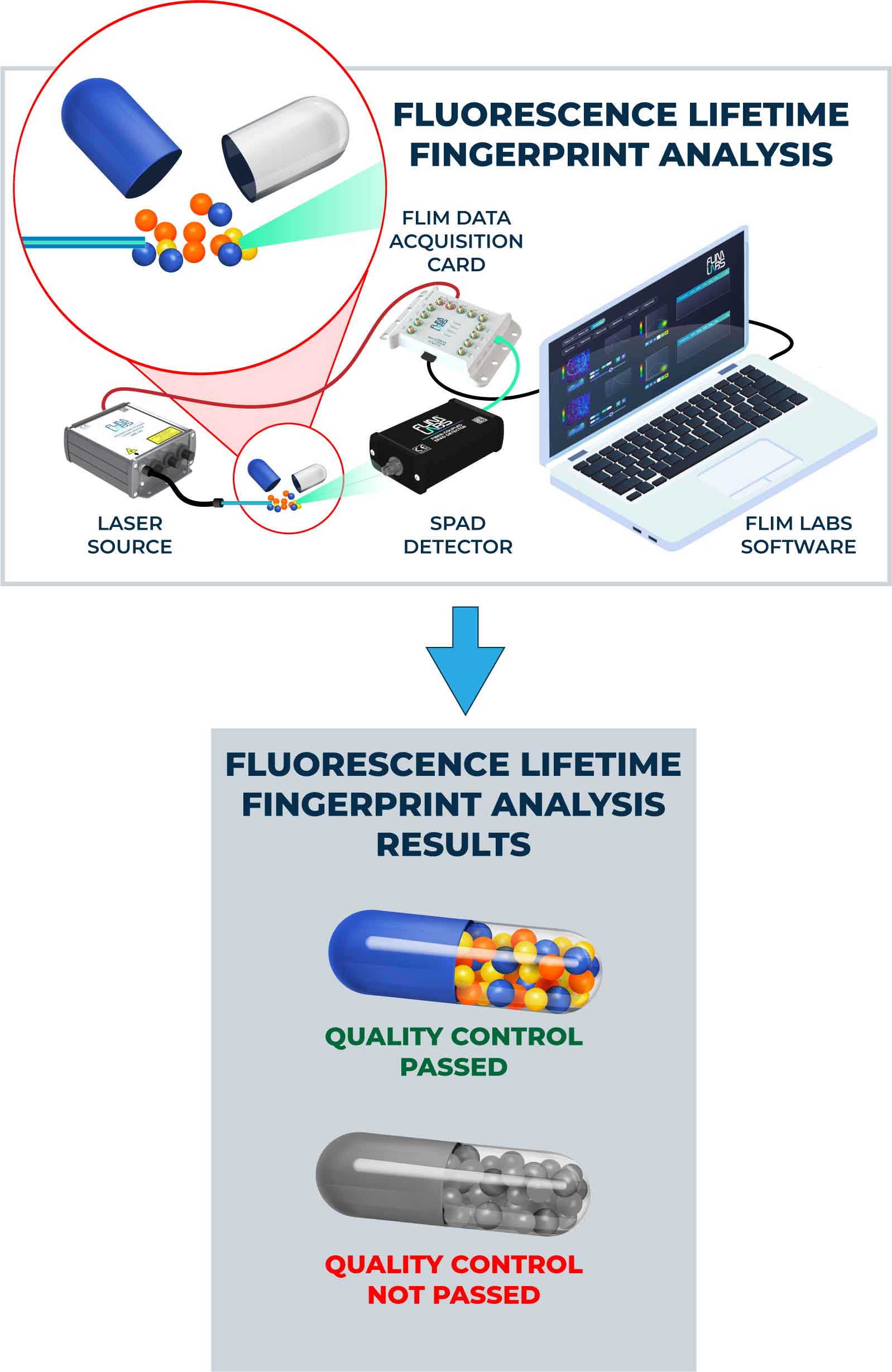
Fluorescence lifetime analysis (FLA) is an essential tool for drug quality control, as it provides sensitive and specific measurements of the properties of fluorophores, which are widely used in the pharmaceutical industry [1]. The technique can be used to measure the lifetime of a fluorophore, which remains constant regardless of environmental factors, making it highly reliable [2]. FLA can be used to assess drug quality in various contexts, including pharmaceutical manufacturing and drug discovery.
One of the advantages of FLA is its sensitivity, which allows the measurement of small changes in the lifetime of a fluorophore. Any change in the lifetime of a fluorophore is directly related to changes in the fluorophore or its environment. Therefore, any changes in drug properties, such as degradation, exposure to light, or temperature, can be readily detected through FLA [3]. This technique can detect even small changes in drug quality that may not be easily detectable using other methods.
FLA also provides high specificity, enabling the differentiation of different drug forms or between different drugs altogether. For example, FLA can distinguish between salt and free base forms of a drug or between drugs with similar structures [4]. This specificity is critical in assessing drug quality, as it enables accurate identification of any drug-related changes.
FLA can also be used to determine the purity of a drug. Impurities in a drug can affect the fluorescence lifetime of the drug, which can then be measured to assess drug purity. By comparing the fluorescence lifetime of a drug to a reference standard, one can determine the purity of the drug [5].
FLA can be used in pharmaceutical manufacturing to assess the quality of raw materials, intermediates, and finished products. It is also useful in drug discovery to assess the stability and quality of lead compounds. In addition, FLA can be used to study the pharmacokinetics of drugs, which is essential for understanding how drugs interact with the body. FLA can be used to study the distribution of drugs in different tissues or compartments of the body [6].
In conclusion, FLA is a highly reliable and sensitive technique for drug quality control. Its sensitivity, specificity, and ability to measure purity make it an ideal technique for assessing the quality of drugs in a variety of different contexts. Moreover, FLA is an important tool for studying the pharmacokinetics of drugs, which is essential for the development of new drugs. The application of FLA in drug quality control and drug development is expected to grow in the future due to its high reliability and sensitivity.
Bibliography
[1] Xie, Y., Liu, X., & Huang, C. (2016). Fluorescence lifetime imaging microscopy for pharmaceutical research. Journal of pharmaceutical analysis, 6(6), 341-348.
[2] Lakowicz, J. R. (2006). Principles of fluorescence spectroscopy (Vol. 3). Springer Science & Business Media.
[3] Nguyen, T. Q., & Hsieh, B. Y. (2017). Fluorescence lifetime imaging of pharmaceuticals. Current Opinion in Chemical Biology, 39, 54-62.
[4] Berezin, M. Y., & Achilefu, S. I. (2010). Fluorescence lifetime measurements and biological imaging. Chemical reviews, 110(5), 2641-2684.
[5] Huang, C., Liu, X., Xie, Y., & Zhu, W. (2014). Pharmaceutical applications of fluorescence lifetime imaging microscopy (FLIM). TrAC Trends in Analytical Chemistry, 62, 1-10.
[6] Vo-Dinh, T., Alarie, J. P., & Cullum, B. M. (2000). Biosensing using fluorescence spectroscopy